Strategic Commitment 2
Harnessing expertise for optimal innovation
Providing an infrastructure to blend clinical and commercial expertise is vitally important in supporting Scotland’s future healthcare innovation priorities and needs. The ability of InnoScot Health to leverage NHS experience and combine this with commercial business skills, which typically sit outside the sphere of traditional healthcare roles, is essential in translating healthcare innovations from original idea to widespread adoption.
InnoScot Health is committed to providing trusted, high quality advice on a pan-Scotland basis, including intellectual property advice, medical device regulation, funding, product development and commercialisation; being responsive to advances across medicine, science, government and NHS policy, legal and regulatory affairs that impact on healthcare innovation process, ensuring the expertise of InnoScot Health remains dynamic and reflective of a modern health landscape, and that Scotland is an internationally recognised health innovation environment combining the best clinical and commercial expertise.
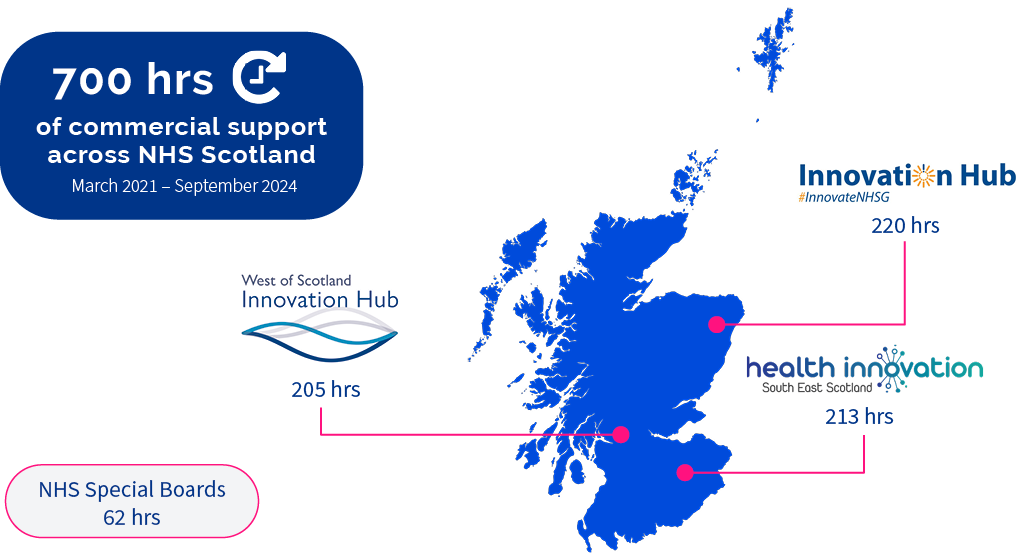
To achieve this, InnoScot Health has offered commercial support to all NHS Boards across Scotland helping to unite specialist innovation skills with triple helix knowledge and ambition. Our regulatory consultancy has equipped innovators with knowledge and confidence to successfully navigate the often complex medical device regulatory landscape; and working collaboratively across the sector an evolving, and diverse pipeline of healthcare innovations are continually progressing.
Scotland-wide commercial support
The commercial support offered by InnoScot Health enables busy healthcare professionals to manage clinical and personal commitments alongside their entrepreneurial ambitions. It also ensures dedicated innovation expertise across intellectual property, funding and investment, project management and commercialisation which is readily available to NHS Boards across Scotland.

Quality Management
InnoScot Health operates an ISO13485:2016 Quality Management System (QMS) — an internationally recognised standard for quality management systems in the design and manufacture of medical devices. This framework ensures all medical device development supported by InnoScot Health is carried out in a way that is compliant with applicable standards and directives.
An overhaul of the QMS system was undertaken by InnoScot Health during 2024, ensuring the organisation is up to date with all the standards, and will form part of continuous improvement. This offers specific benefit to innovators allowing them to demonstrate governance, risk and compliance which will accelerate the design, manufacture and route to market for medical devices.

InnoScot Health has over 20 years of experience and we believe that our ability to bring innovations to market in investor ready form is of significant value.
Gillian Henderson, Head of Project Management, InnoScot Health
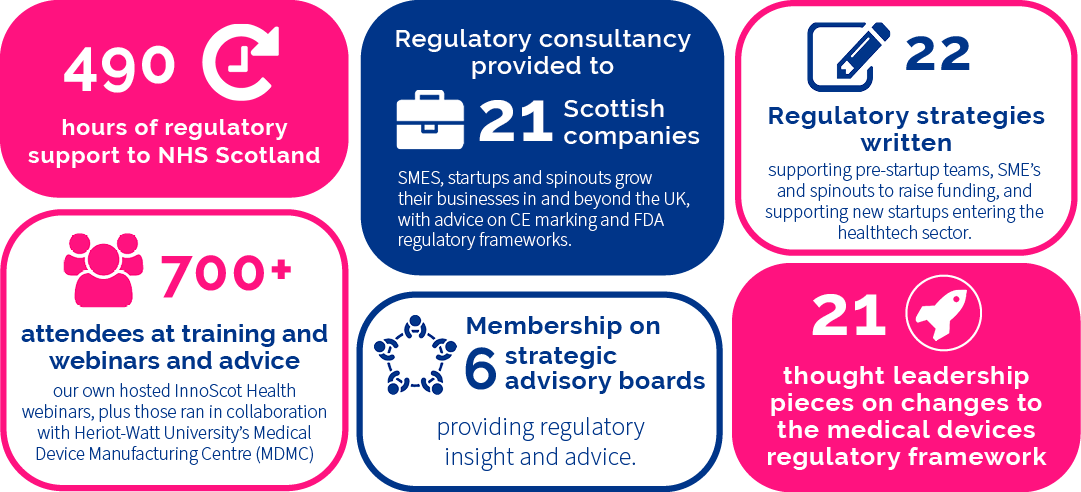
Regulatory Consultancy
Medical devices and diagnostics play a key role in delivering new products that benefit the NHS, the economy and above all, patients. The supply of safe, effective, and innovative medical products is underpinned by a robust regulatory framework.
Provision of consultancy, training and advice to NHS Health Boards, as well as companies or universities working in partnership with NHS Scotland ensures awareness and compliance with medical device regulations and supports the design and development of products under an accredited regulatory framework.

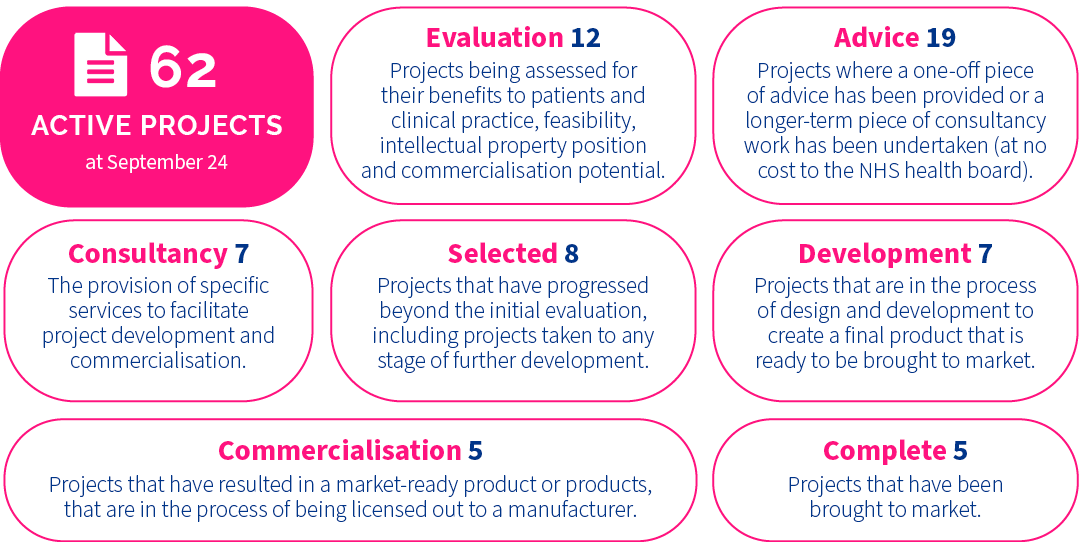
Project Pipeline
InnoScot Health adopts a bespoke approach depending on project requirements. A dedicated Project Manager is allocated to selected projects, acting as the liaison point between InnoScot Health, the innovator, partner NHS Board, and any future commercial partners.
Bespoke support from InnoScot Health includes the protection of NHS Intellectual Property, prototype development and testing, sourcing funding streams, liaison with licence partners and budget impact analysis. The aim is to accelerate ideas along the project pipeline, with the ultimate goal of commercialisation.

Our Contribution to National and Global Priorities
This commitment aligns with Scotland's National Outcomes in the Health, Economy, and Fair Work and Business categories.
It aligns with the United Nations Sustainable Development Goals in the Good Health and Wellbeing; Quality Education; Decent Work and Economic Growth; Industry, Innovation and Infrastructure; Reduced Inequalities; and Partnerships for the Goals categories.
Chat